Securing The US Drug Supply
Although the U.S. drug supply chain remains one of the safest in the world, it is not immune from worldwide threats. Every day, somewhere in the world, comes new news of pirated or otherwise compromised medicine.
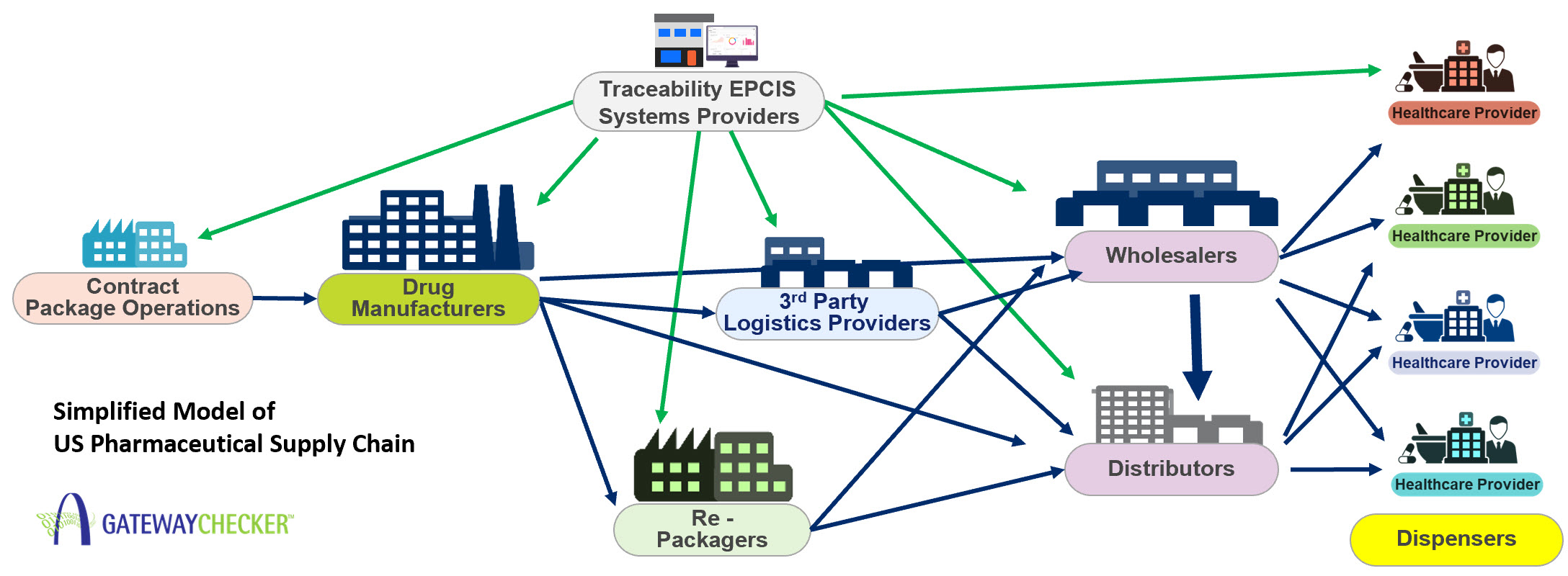
Furthermore, the drug supply chain has become increasingly complex as medicines and raw materials are increasingly being sourced beyond U.S. borders. Threats to the supply chain such as counterfeiting, diversion, cargo theft, and importation of unapproved or otherwise substandard drugs, could result in unsafe, ineffective drugs in the United States.
FDA is committed to ensuring reliable patient access to safe and effective medicines. The full implementation of the Drug Supply Chain Security Act (DSCSA) establishes requirements for the interoperable, electronic tracing of products at the package level.
Traceability of pharmaceuticals is important to ensure the authenticity, safety, security and integrity of the drug supply.
When DSCSA is fully deployed over the next several years, drug traceability shall enable:
- Electronic exchange of information by trading partners at the package level
- Verification of product identifiers at the package level
- Prompt response to suspect and illegitimate products when found
- Improved efficiency of recalls
- Transparency and accountability in the drug supply chain
A key component of enabling traceability is the interoperable exchange of serialized information among trading partners. A common language for supply chain visibility and transparency is necessary to efficiently connect different organizations and information systems.
More than 50 organizations representing leading US Rx manufacturers, wholesalers, retail pharmacies, healthcare providers, government agencies, and industry associations collaborated to forge standards for the interoperable exchange of traceability information (see Applying GS1 Standards for DSCSA and Traceability).
Conforming to these Standards enables implementation consistency among trading partners, aligns data structures, enables common data definitions and avoids deployment delays. Connecting disparate systems without the benefit of standards results in customized data maps which are fragile, error prone, and difficult to maintain.
Adhering to the published GS1 Standards for DSCSA and Traceability enables rapid, robust, reliable connectivity with minimal errors.