Why Conformance Checking Matters?
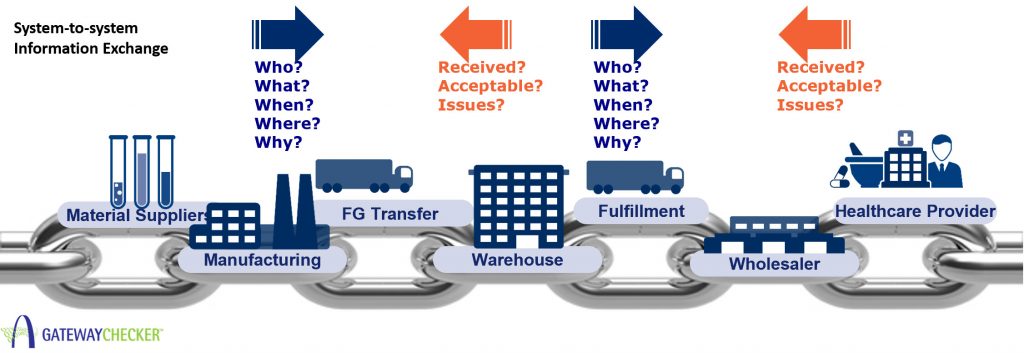
The US pharmaceutical supply chain is made of many different companies that work cooperatively to enable an efficient and effective supply network. The number of system-to-system connections that must receive and transmit supply chain transactions is high.
Even the simplest of drug supply chains requires the establishment, testing and validation of numerous system connections.
At least 3 connections, and perhaps as many as 5 are necessary to effect traceability for even the smallest of pharmaceutical manufacturers. In an industry of 400 – 600 pharmaceutical manufacturers representing more than 20,000 SKUs, one can easily understand the need for standardized approaches to information sharing.
To comply with DSCSA traceability, trading partners must establish connectivity and system interoperability within the pharmaceutical supply chain. Up until now, there was no mechanism to test, evaluate, and score an EPCIS documents conformance to the US healthcare requirements.
Gateway Checker unambiguously assesses and verifies readiness of one system to connect and exchange electronic product code information with another.
Gateway Checker assures conformance:
- Reads, decodes, and verifies EPCIS 1.2 messages per GS1 US Rx Guideline and DSCSA regulations
- Flags and identifies non-conformances; provides error explanations and references to industry guidelines
- Tests and verifies conformance to one of sixteen GS1 pharmaceutical traceability scenarios
- Notifies GS1 of each successfully verified scenario