How To Become Gateway Certified?
Gateway Checker, as a GS1 Certified Conformance Testing Service, can attest to full conformance with the GS1 US Rx EPCIS traceability requirements.
Gateway Checker can quickly, accurately and thoroughly test submitted EPCIS documents. Test submissions either Pass, Pass with Warnings, or Fail. Detailed test results assist users with issue identification, troubleshooting and instructive remedies.
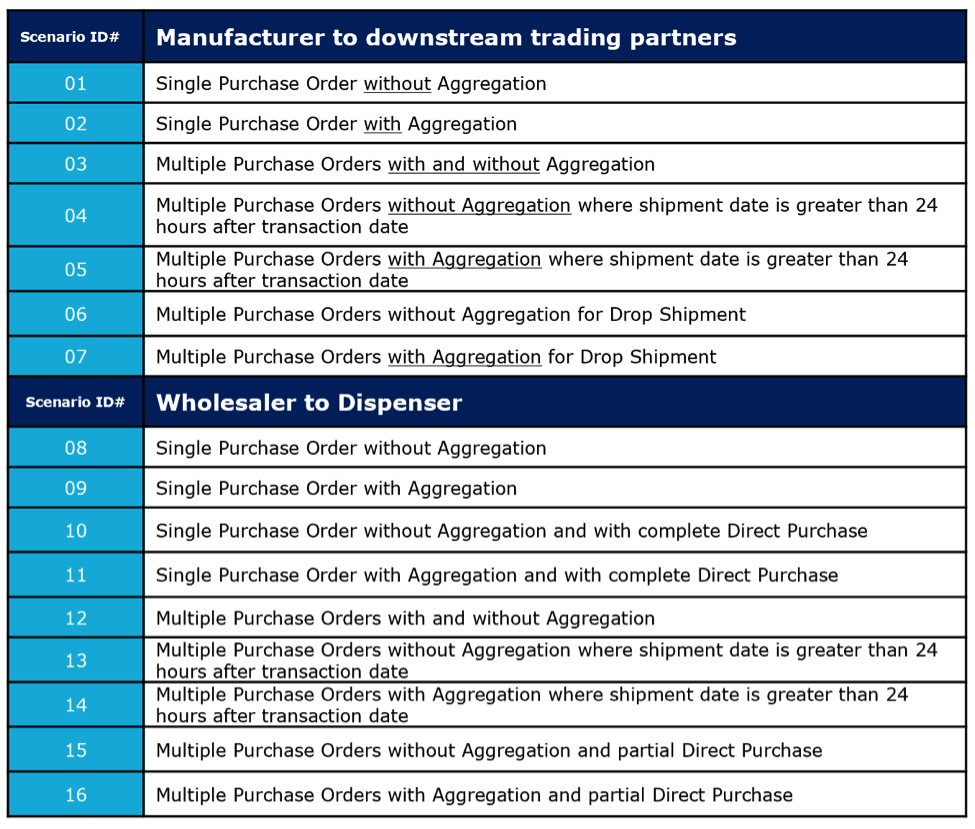
EPCIS documents that pass form, structure, and data content evaluations, are eligible for certification. Conformance testing requires the successful execution of one of 16 pharmaceutical traceability, role-based test scenarios.
Each scenario represents a unique use case of predefined operational considerations, such as supply chain role, aggregated or non-aggregated, number of purchase orders in the shipment, direct purchase, and shipments sent direct to the buyer or drop shipped.
There are 16 GS1 Pharmaceutical Traceability Scenarios covering both manufacturer and wholesaler scenarios. Submitted EPCIS messages that fully conform to the requirements, as evidenced by their ability to generate conforming EPCIS messages for each scenario, are issued a Gateway Certificate.