November 22, 2024
So, You Think You’re Exempt
DSCSA Deadline: Just Days Away The deadline for DSCSA stabilization is just days away, mandating Enhanced Drug Distribution Security (EDDS) requirements for manufacturers, repackagers, wholesale distributors, and dispensers. While the FDA announced an extension from certain components of the FD&C in the form of a phased approach, this extension only applies to certain eligible trading […]
November 22, 2024
What Is Exempt, per recent DSCSA Update
According to the DSCSA Exemption Announcement from the FDA on October 9th, here are the specific components of the legislation that eligible trading partners are exempt from. Section 582(g)(1)(A-F) for Manufacturers and Repackagers, Wholesale distributors, and Dispensers ‘‘(A) The transaction information and the transaction statements as required under this section shall be exchanged in a […]
November 8, 2024
Verification Router Services: A Solution to Drug Shortages?
The Importance of Collaboration to Address Drug Shortages On October 31st, 2024, I listened to a compelling LinkedIn conversation hosted by Chip Davis of the Healthcare Distribution Alliance, titled “Working Collaboratively to End Drug Shortages.” Joined by Laura Bray of Angels for Change and April Gile of the End Drug Shortages Alliance (EDSA), the panelists […]
October 23, 2024
Revisiting Florida’s Plan to Import Drugs from Canada
An FDA-Approved Plan with Major Implications Earlier this year, the FDA authorized Florida’s proposal to import select drugs from Canada under section 804 of the FD&C Act. The law allows companies to import prescription drugs in bulk if doing so saves Americans money and doesn’t put the health and safety of Americans at risk. According […]
October 15, 2024
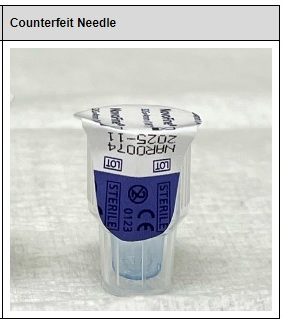
Beware of Counterfeit Ozempic, says FDA
Counterfeiting: A Major Issue with Drastic Consequences On October 2nd, the FDA released an article highlighting the risk of unapproved weight loss drugs, including semgalutide and tirzepatide. These drugs pose a major risk to patients due to their lack of quality assurance. Illegally marketed versions of these drugs have infiltrated pharmaceutical supply chains, most notably, […]